1. OBJECTIVE
The multi drug resistance to several type of antibiotic
agent that causing major problems in the clinic condition. One of the main
causes of multi drug resistance is broad spectrum antibiotics overuse, abuse,
in some cases misuse due to incorrect diagnosis. Antibiotics use in animal
husbandry is also creating some drug resistance bacteria which can be
transmitted to humans. The origin of resistance are said to be start with some
bacteria that said to be have innate resistance against antibiotics and this typically
reflects variation in the structure of
their cell envelope. Resistance may also br phenotypic resulting from
adaption to growth within a specific environment. The origin of antibiotics
resistance genes are unclear however resistance can be achieved by horizontal
acquisition of resistance genes, mobilized via insertion sequences, transposons
and conjugative plasmids by recombination of foreign DNA into the chromosome or
by mutations in different chromosomal loci. There are a number of resistant organisms
causing concern at present. The Gram-positive organisms include methicillin
resistance staphylococcus aureus and coagulase-negative staphylococci,
glycopeptides-intermediate sensitivity s.aureus, vancomycin-resistant
enterococcus species and penicillin-resistant streptococcus pneumonia. Concerns
among the Gram-negative organisms include multidrug-resistant pseudomonas
aeruginosa, stenotrophomonas maltophilia and acinetobacter baumanii and members
of the enterobacteriaceae with extended spectrum β-lactamases. Mycobacterium
tuberculosis and M. avium complex pose major health threat worldwide. [31,
32]
In view to overcome the situation of drug resistance world
is looking towards natural resources. Here the present study aims to review the
potential ability of plant Anacyclus
pyrethrum as antimicrobial. Anacyclus pyrethrum has been used for
many years for its different properties especially for its insecticidal
ability. The present work carried out to prepare different extract of different
part of Anacyclus pyrethrum. This extract has been used for in vitro analysis
of the antibacterial efficacy against various gram +ve and gram –ve
microorganisms. Obtain results evaluated and extract showed maximum
antibacterial efficacy against microorganisms has been taken for MIC and MBC
study. Extract showing maximum efficacy were used to carried out GC/MS for
phytochemical evaluation of Anacyclus
pyrethrum.
2. INTRODUCTION
2.1 PLANT
PROFILE
Pyrethrum belongs to the genus Anacyclus
in the family of Asteraceae. The
flowers of the species Cinerariefolium have long been exploited commercially
for their insecticidal properties. These properties were probably discovered
accidentally in 1840, by a German woman in Dalmatia, who received a bouquet of
flowers on her birthday. After a night long partying, she threw the flowers
into a corner. In the morning the flowers were surrounded by dead insect’s. The
deaths of the insects were associated with the insecticidal properties of the
insects. Since then the flower has undergone extensive research establishing
its complete, effective and safe commercial exploitation as a source of the
natural insecticides collectively known as pyrethrins. [1, 2]
2.2
CLASSIFICATION
Botanical name: - Anacyclus
pyrethrum
Botanical
sources: - Flower, Root, Arial part
Synonym: - Pellitory,
akalakari, akarakara, dalmation
Kingdome: - Plantae
Division: - Spermatophyta
Sub-division:- Angiosperms
Class: - Dicotyledons
Sub class: - Metachlamydae
Order: - Companulate
Family: - Asteraceae
Genus:
- Anacyclus
Species:
- Pyrethrum
2.3 GEOGRAPHICAL SOURCES
Anacyclus
pyrethrum, is a widely-distributed plant known in different countries under
different names Pellitory, akarakara, dalmation etc. Kenya is the
leading producer of pyrethrum extract producing approximately 70% of
the world consumption. Other large producers of pyrethrum are Rwanda, Tanzania
and Tasmania in Australia. In India it is found along the Himalaya,
Jammu and Kashmir, and Bengal.[3,4,5]
2.4 CULTIVATION AND COLLECTION
Pyrethrum is cultivated in tropical zone at an attitude of 1500 to
3500 meters, depending upon the distance from equator. The soil requirements
depend upon rain and other climatic conditions in a particular area. A rain
fall between 800 to 1300 mm consider suitable for pyrethrum cultivation. Since
the plant is very sensitive to frost, sunny periods interrupting rainfall are
desired condition for cultivation. It needs a temperature between 15°C to 25°C.
The seeds are soaked in water and are then wrapped in sacking and buried
in dampens and for four or five days. They are then mixed with dry sand and
sown in well drained, sunny seed bed having carefully plawed, soft sandy soil
which has been freed from stones and clouds. Fertilizer consisting of manure
and superphosphate is worked into the bed before sowing; excessive use of
fertilizer causes too rapid growth and is avoided. One pint of seed is used to
150 square yards of bed; this will yield seedlings enough for an area ten times
as great. After sowing the seeds are covered with earth or ashes, the beds are
shaded with screens with and in periods of drought they are carefully watered.
The seedlings appears in about twelve days, when they are two to three inches
high, fertilizer is added. After 4-5 months the seedlings reach a height of
about four inches and are ready for transplanting. This must be done early
enough to permit the roots to establish themselves firmly before cold weather,
otherwise they will winter kill. The field is carefully plowed weeded, manure
and leveled. The seedlings are planted in rows at intervals of 7 to 12 inches between
rows. The rows are raised or ridged to prevent water collecting around the
roots. If the plants are set too deeply, few flowers are produced. The
harvesting periods extends for 14 to 18 days in given locality and the flower
are picked up when they are about 70% open. The flower picking was done by hands.
Drying generally requires about 5 to 7 days and is accomplished by spreading
the flower heads and root straw mats, in day and placing them indoors at night.
After the flower and root are thoroughly dried so that they can easily be
crumbled between the fingers, they are packed in straw bag and store. Average
yield of the drug per hectare is 300 to 400 kg. [5, 6]
2.5 MACROSCOPIC CHARACTER
It is a perennial, procumbent herb,
resembling chamomile. Stems lie on the ground for part of their length, before
rising erect. Each bears one large terminal flower, the disk being yellow and
the ray’s white, tinged with purple beneath. The leaves are smooth, alternate,
and pinnate, pale green, with deeply cut segments. Fruit obovate achene. The
root is almost cylindrical, very slightly twisted and tapering and often
crowned with a tuft of grey hairs. Externally it is brown and wrinkled, with
bright black spots. The fracture is short, bark with 1-2 circles of resin
ducts, closely adhering to yellowish radiate porous wood in which occur 1-3
rows of resin ducts; odor distinct; taste sweetish, pungent, very acrid,
tingling, sialagogue effect.
Figure 1: Anacyclus Pyrethrum Plant
2.6 MICROSCOPIC CHARACTER
2.6.1 Root - Mature root shows cork consisting of tabular cells, many
of which developed as sclerenchyma; a few inner cork cells contain rosette
crystals of calcium oxalate; secondary cortex consisting of isodiametric or
tangentially, elongated, thin-walled, parenchymatous cells, a few
sclerenchymatous cells also found scattered in secondary cortex; secondary
phloem consisting of usual elements, cambium 2-5 layered, secondary xylem very
wide consisting of xylem vessels, tracheids and xylem parenchyma; vessels
pitted, more or less in groups distributed throughout xylem, more and wider
vessels found towards peripery, xylem fibers thick-walled, 1.37-28.8 μ in
width, 53.2 - 231 μ in length having narrow lumen, medullary
rays numerous, running straight, bi to tri and multiseriate, uniseriate rays
very rare, starting from primary xylem and reaching up to secondary cortex; ray
cells thick-walled, radially elongated, inulin.
Figure 2: Photomicrograph of cross section of
root: A- cork, B-Ring of stone cells,
C-Parenchyma of primary cortex,
D-Cambian, E-Medullary ray, F-Xylem
present in cells of secondary cortex, secondary phloem and
medullary rays; oleo-resinous schizogenous glands found scattered in secondary
cortex, secondary phloem and medullary rays; calcium oxalate crystals in
rosette form present in secondary cortex, secondary phloem, secondary xylem and
medullary ray cells.[7],[8]
2.6.2 Powder - Ash colored; shows vessels having scalariform thickening,
rosette crystals of calcium oxalate and fragments of sclerenchyma; also gives
positive tests for inulin.
2.7 PHYTOCHEMICAL CONSTITUENTS
OF ANACYCLUS PYRETHRUM
2.7.1 Flower:
-
Anacyclus pyrethrum flower’s
principles are located in the oleoresin secretion of floral parts of partially
open or closed flowers. Although pyrethrin I and pyrethrin II are the main
active constituents, it also contains other active compounds called cinerin I, cinerin
II, jasmoline I and Jasmoline II. All these constituents are chemically esters.[5]
2.7.2 Root: - Root contains alkyl amides,
which active constituent’s pyrethrin. Alkyl
amide fraction of roots of Anacyclus pyrethrum is made up of the following
isobutylamides and tyramine amides. The root contain Anacyclin, Pellitorine
enetriyne alcohol (pyrethrins ), hydrocarolin, inulin (50%), traces of volatile
oil and (+)-sesamin. They also contain N-(2-P-hydroxy phenylethyl) deca,dodeca,
and tetradeca- trans-2,a new series of tyra mine amides corresponding to the
isobutylamides.[9]
2.7.3 Arial part: - Anacyclus pyrethum arial parts contain active constituent is
Anacyclin, N-methylanacyclin, Nmethyl- N-(2-methyl propyl) 2, 8-decadiene 4,
6-diynamide along with very low quantity of pyrethrin I and pyrethrin II.[9] Amongs
others Anacylcus herb contains various chemical constituents such as
pellitorine, anacyclin, enetriyne alcohol, inulin, hydrocarolin, sesamin and
traces of volatile oils. Pyrethrin, an alkaloid is considered to be an active
constituent in this herb. The roots of the herb is rich in rubefacient and
stimulant properties. Various medicinal benefits of the herb are discussed in
this article.
2.7.4 Medicinal uses
Pyrethrum plant is used for various medicinal the main used are
enlisted as:
1. The powdered root forms a good snuff to
cure chronic catarrh of the head and nostrils and to clear the brain, by
exciting a free flow of nasal mucous and tears.
2. Pyrethrum kills
insect by disrupting their nervous systems. Pyrethrins are toxic to the “sodium
channel” the cellular structure that allows sodium ions to enter a cell as part
of the process of transmitting a nerve impulse.
3. Pyrethrum root used almost exclusively
as a sialagogue in headache, neuralgic and rheumatic affections of the face,
toothache, etc., or as a local stimulant in epalsy of the tongue or throat, or
relaxation of the uvula.
4. Pyrethrum
contains anacycline, isobutylamide, inulin and a trace of essential oil. Use of
the drug in patients with insulin
dependent diabetes mellitus reduces the dose of insulin. It decreased the
plasma glucose and serum cholesterol levels after oral administration for 3–6
weeks.
5. Ues to kill lice and their eggs and in
mosquito coil.[10], [11], [12
2.7.5 Ayurvedic Properties
1 Guna (Properties) – Ruksha (Dry),
Tikshan (Sharp)
- Virya
(Potency) – Ushan (Hot)
- Rasa
(Taste) – Katu (Pungent)
- Prabhav
(Impact) – Shukrashodhan
- Karma
: Vatahara, Pittahara, Kaphahara, saukrala, Vajikara, Svedakara, Dipana, Buddhivardhaka,
Balakrka.[7]
3.
Material and Method
1
Test
plant: leaves / root /whole plant powder
2
Solvents : Ethanol & Methanol (500 ml each)
3
Glassware: Conical flasks -
50ml /8nos, Beakers (8nos.),Petri dish/cover ,Glass rod
4
Measuring
cylinders
5
Cotton
6
Whattman filter paper no.1
7
Muller Hinton agar/Nutrient agar/Nutrient
Broth medium
8
Test organisms: Staphyllococcus aureus ,E.coli, Pseudomonas aeruginosa
, Salmonella abony , Proteus vulgeris.
3.1 LIST OF CHEMICALS AND INSTRUMENTS
3.1.1 List
of Chemicals
Table No. 1: List of Chemicals
Sr. No.
|
Chemicals
|
Manufacture
|
1.
|
Readymade Agar Media
|
Hi-Media
|
2.
|
Beef extract
|
Hi-Media
|
3.
|
Peptones
|
Hi-Media
|
4.
|
Ethanol
|
Rankem
|
5.
|
Methanol
|
Rankem
|
6.
|
Sodium Chloride
|
Merk
|
3.1.2. List of instruments
Table No.2: List of Instrument
Sr. No.
|
Instruments
|
Manufacturer
|
1.
|
Laminar
Air Flow
|
Veekay Blower Industries, India.
|
2.
|
Autoclave
|
D4 Surgicals India
|
3.
|
GC/MS
|
SHIMADZU Japan
|
4.
|
BOD
Incubator
|
Vertical
Industries, India
|
5.
|
pH meter
|
Orion 4 star
|
6.
|
Analytical balance
|
Sartorius BP 210
|
7.
|
Microbial
Spreader
|
-
|
8.
|
Cork Borer
|
-
|
3.2. LIST OF TEST
MICROORGANISM
Table No.3: Test
Microorganism
Sr.No.
|
Microorganisms
|
ATCC NO.
|
1.
|
Staphyllococcus
aureus
|
6538
|
2.
|
Escherichia
coli
|
10346
|
3.
|
Pseudomonas
aeruginosa
|
9027
|
4.
|
Salmonella
abony
|
6014
|
5.
|
Proteus
vulgeris
|
6380
|
(All cultures were 18hrs old.)
All the bacterial culture used for antimicrobial analysis was
old. The bacteria were maintained on nutrient
broth (NB) at 37°C.
3.5 PREPARATION
OF INOCULUM
The
gram positive S.aureus and gram negative E.coli, Proteus and Psedomonas were
subculture in nutrient broth and kept overnight in oven at temperature of 37 °C.
For subculture 7-10 days old culture were used. The subculture microorganisms
are used in antimicrobial study.
3.6 Preparation
of the Plant Material & Extraction
The
whole plant of the Anacyclus pyrethrum was collected
from Lord’s Herbal and Medicinal plant centre, Maharashtra and authticated. The completely
dried parts washed
with distilled water & cut into pieces.
Dried
roots were crushed into molten and pestle 10 gms of powder
were added worth 100 ml of methanol & ethanol each.
The flask was closed
with cotton plugs & incubated at room temperature (30 deg. Celsius) for 24
hours. Extract was filtered using Whatman filter paper no.1.
Label the extract as TS1, TS2 respectively. The dried powder
of aerial part of the plants was also mix with 100ml of methanol & ethanol each. The
mixture was incubated for 24 hours at room temperature (30 deg. Celsius).Now the
extract was filtered using Whatman filter paper no.1 and
label the extract as TS3, TS4 respectively.
4.7
Antimicrobial assay
Agar well diffusion assay method was
used for determining the zone of inhibition. 25ml of nutrient agar was added in
the Petri dish, the nutrient agar was allowed to settle for 10-15 minutes. Now
punch the agar with the cork borer (10mm). Add 0.1ml of test microorganism on
the plate and spread with a sterile spreader. Now load the extracts in the
wells (0.1ml).Allow the plates for incubation for 24hrs at 37˚C.
Four
test samples were used for antibacterial assay. Antibacterial activity
determined by the agar well diffusion method. Agar cup bioassay was employed for
testing antimicrobial activity of plant extract. The ready-made agar medium
(Hi-media, 39g) was suspended in distilled water and autoclaved at pressure of
15 lb/inc2 for 20 minutes. Test organisms are
inoculated in nutrient broth and incubated at 37ᵒc for 18-24 hours are used.
10µl test organism was used to inoculate agar plate with help of sterile
spreader uniformly and wait for 5-6 minutes after inoculation allow the liquid
culture to soak into the agar. Then four wells were made on the surface of the
agar plate by the use of sterile cork borer and named as set-1 i.e TS1, TS3,
-ve(methanol) and antibiotic or set-2 i.e TS2, TS4, negative control (ethanol)
and antibiotic. Now with help of sterile micropipette 10 µl of methanolic or
ethanolic extract was added in wells. Remaining wells were filled with10 µl of
negative control (Methanol or Ethanol) and 10 µl of standard antibiotic. Wait
for 5-6 minutes after inoculation to allow the liquid extract to soak into the
agar. After that plates are incubated at 37ᵒC for 24-48 hours and zone of
inhibition were measured. Five replicates were maintained for each treatment and the
result of average ranges is documented in the result section.
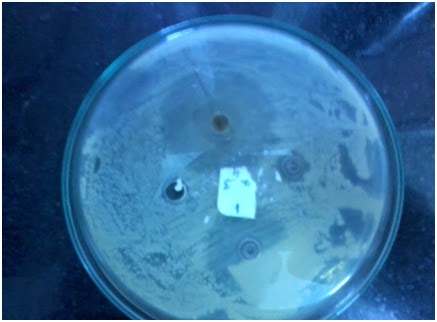
Figure 3: ZonE of
inhibition of E.coli, 1-ts2, 2-ts-4, 3 -ethanol 4 - antibiotic
Figure 4: Zone of
inhibition of PSEUDOMONAS, 1- ts2, 2- ts-4, 3- ethanol 4- antibiotic.
Figure 5:zone of
inhibition of s.aureus,1- ts2, 2- ts-4, 3- ethanol 4- antibiotic
3.8 Minimum Inhibitory Concentration (MIC):-
Minimum inhibitory concentration is defined as the
lowest concentration of an antimicrobial that will inhibit the visible growth
of microorganism after overnight incubation. Minimum inhibitory concentration
is used by diagnostic laboratories mainly to confirm resistance but most often
as research tool to determine the minimum inhibitory concentration breakpoints.
There are two method of MIC testing i) Broth dilution method and ii) Agar dilution
method.
Here broth dilution method was used to determine the
MIC of TS1, TS2, TS3, TS4. For MIC sterile graduated pipettes of 5ml, 2ml and 0.5ml,
small capped test tubes and overnight broth cultures are required. Six sterile
caped test tube taken and labeled as A,B,C,D,E,F to each tube 4ml of freshly prepared broth
added follow by addition of 0.5 ml of
overnight grown broth culture of E.coli having turbidity equivalent 0.5 McFarland. Different concentration of anacyclus
pyrethrum was prepared by serial dilution method with given strength of 10,000
µl/ml. Five sterile test tube taken and labeled as 1,2,3,4,5 to each tube add
4.5ml of freshly prepare broth. Now transfer 0.5ml of stock solution of
Anacyclus pyrethrum to test tube no.1 this will give the concentration of 500µl/ml.
Similarly different concentration are prepared 50 µl/ml, 5 µl/ml, 0.5 µl/ml,
0.05 µl/ml by following-
Add 0.5 ml of test tube no.1 to test tube no.2 -
(50µl/ml).
Add 0.5ml of test tube no.2 to test tube no.3 -
(5µl/ml).
Add 0.5 ml of test tube no. 3 to test tube no.4 -
(0.5µl/ml).
0.5ml of different concentration of Anacyclus
pyrethrum from test tube1, 2, 3, 4 added to A, B, C, D tube respectively and to
tube F 0.5ml of freshly prepared broth added as a control. Incubate the test tube
for 24 hours and results are documented.
3.9 Minimum Bactericidal Concentration
Minimum bactericidal concentration is the lowest
concentration of antimicrobial that will prevent growth of an organism after
subculture on to antibiotic free media.0.5 ml of test tube 1,2,3,4 are poured
into freshly prepared agar plate separately and incubated overnight. After
incubation the plate showing no growth of organisms are recorded as MBC .
3.10 GAS CHROMATOGRAPHY-MASS SPECTROSCOPY (GC-MS)
ANALYSIS:-
The
GC-MS is composed of two major building blocks: the gas chromatograph
and the mass spectrometer.
The gas chromatograph utilizes a capillary column which depends on the column's
dimensions as well as the phase properties. The difference in the chemical
properties between different molecules
in a mixture will separate the molecules as the sample travels the length of
the column. The molecules are retained by the column and then elute (come off
of) from the column at different times (called the retention time), and this
allows the mass spectrometer downstream to capture, ionize, accelerate,
deflect, and detect the ionized molecules separately. The mass spectrometer
does this by breaking each molecule into ionized
fragments and detecting these fragments using their mass to charge ratio. These
two components, used together, allow a much finer degree of substance
identification than either unit used separately. It is not possible to make an
accurate identification of a particular molecule by gas chromatography or mass
spectrometry alone. The mass spectrometry process normally requires a very pure
sample while gas chromatography using a traditional detector (e.g. Flame Ionization
Detector) cannot differentiate between multiple molecules that happen to take
the same amount of time to travel through the column (i.e. have the same
retention time) which results in two or more molecules to co-elute. Sometimes
two different molecules can also have a similar pattern of ionized fragments in
a mass spectrometer (mass spectrum). Combining the two processes reduces the
possibility of error, as it is extremely unlikely that two different molecules
will behave in the same way in both a gas chromatograph and a mass
spectrometer. Therefore, when an identifying mass spectrum appears at a
characteristic retention time in a GC-MS analysis, it typically lends to
increased certainty that the analyte of interest is in the sample.
GC-MS analysis of the samples was carried out using
Mass Sensitive Detector, equipped with a cross linked methyl silicone gum phase
capillary column (25m x 0.32mm). Hydrogen was used as the carrier gas and the
temperature programming was set with initial oven temperature at 40°C and held
for 2 min and the final temperature of the oven was 280°C with rate at 10°C/
min. A 2 μL sample was injected with splitless mode. Mass spectra were recorded
over 35-650 amu range with electron impact ionization energy 70 eV. The total
running time for a sample is 45 min.
4.
Result and DISCUSSION
4.1 Preparation of the Plant MATERIAL & EXTRACTION
Four samples were
subject for extraction and the resulted extract labeled as the TS 1 TS2 TS3 TS4
respectively. TS1 and TS3 are methanolic extract and TS2 and TS4 are ethanolic
extract of AP. TS1 and TS2 are root part of AP where as TS3 and TS4 are aerial.
These extracts were analyzed for their antibacterial effects immediately after
their preparation.
4.2
Antimicrobial assay
Effects of different extract of Anacyclus pyrethrum
were tested against five different microorganisms. All extract inhibited the
microorganism with varying degree of sensitivity. Result obtained in the present study relieved that the tested medicinal
plant extract posses potential antibacterial activity against S. aureus,
E.coli, Pseudomonas aeruginosa, Proteus vulgeris. When tested by well diffusion
method by using methanolic and ethanolic extract of Anacyclus pyrethrum.
Table 4: Antimicrobial activity of Methanol extract of Anacyclus Pyrethrum
Micro-organism
|
Test
Solution 1
|
Test
Solution 3
|
Methanol
( -ve )
|
Antibiotic
|
S. Aureus
|
12 mm
|
10 mm
|
8 mm
|
28 mm
|
E. Coli
|
18 mm
|
10 mm
|
8 mm
|
30 mm
|
Pseudomonas
|
16 mm
|
14 mm
|
10 mm
|
28 mm
|
S.Abony
|
14mm
|
12mm
|
8mm
|
28mm
|
Proteus
|
18 mm
|
12 mm
|
8 mm
|
30 mm
|
The methanolic extract of
root (TS1) showed significant activity against E.coli and Proteus, around 18mm,
the highest antibacterial and least activity recorded in S. aureus measured 12
mm. Methanolic extract of aerial part of Anacyclus pyrethrum (TS3) exhibit low
activity as compare to TS1.the highest activity of TS3 is for Pseudomonas 14 mm
and lowest in E.coli and s. aures 10mm.
The antibacterial activity was more for TS1 as compared to TS2. The diameter of
inhibition zones ranged from 10 mm to 18 mm among different microorganism species
and increased with the increase in concentration of test solution. The maximum
zone of inhibition was found for E.coli, Proteus with TS1 and minimum zone of
inhibition found to for 10mm for TS3.
Figure 7: Effect of Methanol Extract of AP on Microbes
Table 5: Antimicrobial activity of Ethanol extract of Anacyclus Pyrethrum
Micro-organism
|
Test Solution 2
|
Test
Solution 4
|
Ethanol
( -ve )
|
Antibiotic
|
S. Aureus
|
16
mm
|
15 mm
|
8 mm
|
28 mm
|
E. Coli
|
26
mm
|
18 mm
|
8 mm
|
29 mm
|
Pseudomonas
|
24 mm
|
20 mm
|
8 mm
|
28 mm
|
S.Abony
|
14 mm
|
12 mm
|
8 mm
|
28 mm
|
Proteus
|
23 mm
|
22 mm
|
8 mm
|
30 mm
|
Ethanolic extract of root
(TS2) and aerial part (TS4) showed significant activity against S. aureus,
E.coli Pseudomonas aeruginoisa, S. abony and Proteus. Ethanolic extract of root
TS2 show around 26mm, the highest antibacterial with 24mm and 23mm respectively
for Pseudomonas and Proteus and least activity recorded in S. abony measured 14
mm. Ethanolic extract of aerial part of Anacyclus pyrethrum (TS4) exhibit low
activity as compare to TS2.The highest activity of TS4 is for Proteus 22 mm and
lowest in S. abony 12 mm . The antibacterial activity
was more for TS2 as compared to TS4. The diameter of inhibition zones ranged
from 12 mm to 26 mm among different microorganism species. The maximum zone of
inhibition was found for E.coli, with TS2 and minimum zone of inhibition found
to for 12 mm for TS4.
Figure 8: Effect of Ethanol extract of AP
on Microbes
Overall Ethanolic extract of root and aerial part
posses more antibacterial potency as compare to methanolic extract of root and
aerial part of AP. Among methanolic and ehthanolic extract, root extracts shows
more antibacterial potency as compare to aerial part extract. Ethanol extract
of root and aerial part posses more potency as compare to methnolic extract of
root and aerial part. Hence among alls ethanolic extract of root (TS3) shows
good antimicrobial activity with maximum potency against E.coli. Minimum
activity was observed for Methanolic extract of aerial part of anacyclus
pyrethrum against S. aureus and E.coli. From these data it is clear that
ethanolic extracts are more potent antimicrobials as compare to methanolic
extracts. It also indicate that root part of AP is posses more antimicrobial
potency as compare to aerial part of AP. As the maximum antimicrobial activity
is posses by TS3 against E.coli it is consider for further investigation.
4.3 Minimum Inhibitory Concentration (MIC)
0.5ml of
different concentration of Anacyclus pyrethrum from test tube1, 2, 3, 4 added
to A, B, C, D tube respectively and to tube F 0.5ml of freshly prepared broth
added as a control. Incubate the test tube for 24 hours and results and
documented the minimum concentration showing clear solution are observed and
recorded as MIC that is 500µl/ml overnight grown culture of test tube are used
for minimum bactericidal concentration.
Table 6: Minimum Inhibitory concentration of TS2 against E. coli
Microorganism
|
Minimum
inhibitory Concentration of TS2
|
E.coli
|
500µl/ml
|
4.4 Minimum Bactericidal Concentration
Minimum bactericidal concentration is the lowest
concentration of antimicrobial that will prevent growth of an organism after
subculture on to antibiotic free media.0.5 ml of test tube 1,2,3,4 are poured
into freshly prepared agar plate separately and incubated overnight. After
incubation the minimum concentration containing plate showing no growth of
organisms are recorded as MBC.
Table 7: Minimum Bactericidal concentration of TS2 against E.coli
Microorganism
|
Minimum
Bactericidal concentration of TS2
|
E.coli
|
50µl/ml
|
4.5
THIN LAYER CHROMATOGRAPHY (TLC)
Chromatography technique used to separate
the mixtures of constituents. Thin layer chromatography was performed on a sheet of glass aluminum foil,
which was coated with a thin layer of adsorbent
material silica gel. This layer of adsorbent is
known as the stationary phase.
After the sample has been applied on the plate with the help of capillary tube,
a solvent mixture of Toluene: ethyl acetate ( 85:15 ) is drawn up the plate via
capillary action. Because different analytes ascend
the TLC plate at different rates, separation is achieved. This separated
analytes are cut and dissolve in ethanol and use for GC-MS analysis.
Figure 9: TLC of TS2
4.6
GC-MS ANALYSIS
Gas
chromatography-mass spectrometry (GC-MS) analysis of the PEE of A. pyrethrum
were carried out in order to characterize the extract .GC-MS of the PEE
indicated the presence of a compound with the fragmentation of which matched
with compounds enlisted below
Analytical
conditions maintained were as follow
temperatures:
|
250°C (column)
250°C (injector) 300°C (detector) |
column gas flow:
|
1.2 mL/min (hydrogen)
|
make-up gas flow:
|
60 mL/min (nitrogen)
|
injection size:
|
1.0 µL
|
injection split:
|
55 to 1
|
column:
|
SPB-l, 1.0-µm film thickness, 30 m ×
0.32-mm i.d. fused silica (Supelco Inc.)
|
retention times:
(min) |
2.38 (p-chlorobiphenyl)
16 (cinerin I) 7.31 (jasmolin I) 12.83 (cinerin II) |
Figure 10: GC/MS Analysis
Compound
|
CAS no.
|
R1
|
R
|
pyrethrin I
|
[121-21-1]
|
CH3
|
CH2CH=CHCH=CH2
|
pyrethrin II
|
[121-29-9]
|
COOCH3
|
CH2CH=CHCH=CH2
|
cinerin I
|
[25402-06-6]
|
CH3
|
CH2CH=CHCH3
|
cinerin II
|
[121-20-0]
|
COOCH3
|
CH2CH=CHCH3
|
jasmolin I
|
[4466-14-2]
|
CH3
|
CH2CH=CHCH2CH3
|
jasmolin II
|
[1172-63-0]
|
COOCH3
|
CH2CH=CHCH2CH3
|
The pyrethrins are a naturally-occurring group of six
chemically-related esters, each of which is insecticidally active. Three (
pyrethrins I) are esters of chrysanthemic acid, and three (pyrethrins II) of
pyrethric acid. The alcohol moieties are pyrethrelone in pyrethrin I and II,
cinerolone in cinerin 1 and 2, and jasmolone in jasmolin 1 and 2. Table 1 gives
the structures of the acid and alcohol moieties.
Table 8: Components moieties of pyrethrin
esters
ACID
|
ALCOHOL
|
|
|
|
|
|
|
|
|
The six pyrethrin esters are designated collectively by the
ISO common name “pyrethrins”. Pyrethrin 1 predominates. Information on the
individual esters is provided below, where the IUPAC chemical names are
according to Rothamsted nomenclature
4.6.1. Pyrethrin I
Chemical names:-
IUPAC:
(Z)-(S)-2-methyl-4-oxo-3-(penta-2,4-dienyl)cyclopent-2-enyl (1R)-
trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
CAS: [1R-[1α[S*(Z)],3β]]-2-methyl-4-oxo-3-(2,4-pentadienyl) cyclopenten-1-yl
2,2-dimethyl-3-(2-methyl-1-
propenyl)cyclopropanecarboxylate
CAS No.: 121-21-1
Molecular formula: C21H28O3 Molecular weight: 328.4
Pyrethrin I
|
Figure
11: Structure of
Pyrethrin I
4.6.2. Cinerin 1
Chemical names:-
IUPAC:-
(Z)-(S)-3-(but-2-enyl)-2-methyl-4-oxocyclopent-2-enyl
(1R)-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
CAS:-[1R-[1α[S*(Z)],3β]]-3-(2-butenyl)-2-methyl-4-oxo-2-cyclopenten-1-yl2,2
dimethyl-3-(2-methyl-1-propenyl) cyclopropanecarboxylate
CAS No.:- 25402-06-6
Molecular formula: C20H28O3
Molecular weight: 316.4
Cinerin I
|
Figure 12: Structure of Cinerin I
4.6.3 Jasmolin 1
Chemical names:-
IUPAC: (Z)-(S)-2-methyl-4-oxo-3-(pent-2-enyl)cyclopent-2-enyl
(1R)-trans-2,2-dimethyl-3-(2-methylprop-1-enyl) cyclopropanecarboxylate
CAS: [1R-[1α[S*(Z)],3β]]-2-methyl-4-oxo-3-(2-pentenyl)-2-cyclopenten-1-yl
2,2-dimethyl-3-(2-methyl-1-propenyl) cyclopropanecarboxylate
CAS No.:- 4466-14-2
Molecular formula: C21H30O3 Molecular weight: 330.4
|
Jasmolin I
|
Figure
13: Structure of Jasmolin I
5.6.4. Pyrethrin II
Chemical names:-
IUPC: - (Z)-(S)-2-methyl-4-oxo-3-(penta-2, 4-dienyl)cyclopent-2-enyl
(E)-(1R)-trans-3-(2-methoxycarbonylprop-1-enyl)-2, 2-dimethylcyclopropanecarboxylate
CAS:-[1R-[1α[S*(Z)], 3β (E)]]-2-methyl-4-oxo-3-(2,4-pentadienyl)-2-cyclopenten-1-yl
3-(3-methoxy-2-methyl-3-oxo-1-propenyl)-2, 2-dimethylcyclopropanecarboxylate
CAS No.: 121-29-9
Molecular formula: C22H2805 Molecular weight: 372.4
Pyrethrin II
|
Figure 14: Structure of Pyrethrin II
5.6.5 Cinerin II
Chemical names:-
IUPAC: (Z)-(S)-3-(but-2-enyl)-2-methyl-4-oxocyclopent-2-enyl
(E)-(1R)-trans -3-(2 methoxycarbonylprop-1-enyl)-2,2-dimethylcyclopropanecarboxylate
CAS: [1R-[1α[S*(Z)], 3β
(E)]]-3-(2-butenyl)-2-methyl-4-oxo-2-cyclopenten-1-yl 3-(3-methoxy-2-methyl-3-oxo-1-propenyl)-2,2-dimethyl
cyclopropanecarboxylate
CAS No.: 1172-63-0
Molecular formula: C21H28O5 Molecular weight: 360.4
|
Cinerin II
|
Figure
15: Structure of Cinerin II
5.6.6 Jasmolin II
Chemical names:
IUPAC: (Z)-(S)-2-methyl-4-oxo-3-(pent-2-enyl)cyclopent-2-enyl
(E)-(1R)-trans-3-(2 methoxycarbonylprop-1-enyl)-2,2-dimethylcyclopropanecarboxylate
CAS: [1R-[1α[S*(Z)],3β(E)]]-2-methyl-4-oxo-3-(2-pentenyl)-2-cyclopenten-1-yl 3-(3-methoxy-2-methyl-3-oxo-1-propenyl)-2,2-dimethylcyclopropanecarboxylate
CAS
No.:
121-20-0
Molecular formula: C22H30O5
Molecular weight: 374.4
Jasmoline II
|
Figure
16: Structure of
Jasmoline II
5. conclusions
Emergence
of multi-drug resistance in human and animal pathogenic bacteria as well as
undesirable side effects of certain antibiotics has triggered immense interest
in the search for new antimicrobial drugs of plant origin. Some strains of bacteria
were found to be sensitive to the plant extracts but showed resistant against
antibiotics. Plants are important source of potentially useful structures for
the development of new chemotherapeutic agents. The first step towards this
goal is the in vitro antibacterial
activity assay. Many reports are available on the antiviral, antibacterial,
antifungal, anthelmintic, antimolluscal and anti-inflammatory properties of
plants. Some of these observations have helped in identifying the active principles
responsible for such activities and in the developing drugs for the therapeutic
use in human beings. However, not many reports are available on the
exploitation of antimicrobial property of plants for developing commercial
formulations for applications in crop protection. In the present study, the
methanol and ethanol extract of root and
aerial part of plant Anacyclus pyrethrum showed the activity
against S. aureus, E coli, Pseudomonas aeruginosa, Salmonella abony and Proteus vulgeris and Plant based products
have been effectively proven for their utilization as source for antimicrobial compounds.
For instance, both extracts of Anacyclus pyrethrum exhibited inhibitory
extracts of root and aerial part of Anacyclus pyrethrum were evaluated
by the well diffusion method against five S. aureus, E coli, Pseudomonas
aeruginosa, Salmonella abony and Proteus vulgeris. Methanol extract of
aerial and root of Anacyclus pyrethrum exhibits
microbial growth on agar media but ethanol extract of aerial and root extract
showed more potent antimicrobial activity.
The
traditional use of Anacyclus pyrethrum
against enteric pathogens proves the best in particular to the E.coli. The co
relation and the exact mode of these active components as a potential novel
entities need to be explore in future research.
The results of present investigation clearly
indicate that the antibacterial and antifungal activity vary with the species
of the plants and plant material used. Thus, the study ascertains the value of
plants used in Ayurveda, which could be of considerable interest to the development
of new drugs.
7. References
1.
Casida JE, Quisdad GB, Pyrethrum Flowers,
Production, Chemistry, Toxicology, and Uses, Oxford University Press, New York,
1995; 46-53.
2.
Casida JE, Pyrethrum, The Natural Insecticide,
Academic Press, New York 1973; 3: 43-45.
3.
Lloyd UJ, History of the vegetable drug’s U.S.P 1911.
4.
Kenya’s Pyrethrum Industry published by Ministry of
Agriculture and Rural development of Keneya, 2005:1-10.
5.
Kokate CK, Porohit AP, Gokhale SB, Pharmacognosy, Nirali
Prakashan, New Delhi Edittion 33, 2007; 578-581.
6.
Gnadinger CB, Pyrethrum
flower, Biotech Publisher, Spain, 2001; 1: 1-34.
7.
The Ayurvedic pharmacopeia of India, part- I, Volume-II,
1999; 1:1-2.
8.
Lamnuer D and Kamal B., A guide to medicinal plant In North
Africa, Anacyclus Pyrethrum, IUCN Mangla, 2005:35-37
9.
The wealth of India “A dictionary of Indian raw material and
Industrial product, 1948; 9:250-254.
10.
Ursulla B., Federal biology researches center for
agriculture and forestry, Germany, 2002; 1: 685-749.
11.
Odinga WA and Angedu CA, The relationship between Pyrethrin
and the yellow pigmentation in pyrethrum, African journal of science and technology
science and engineering series, 2003;4(2):116 –123.
12.
Insecticidal Factsheet, Journal of pesticide reform, Spring,
2002; 22(1): 14-15.
13.
Tattesfield F,
Pyrethrum Post, Official publication of Pyrethrum board of keneya 1948; 1: 1-2.
14.
Casida JE; Pyrethrum flowers and Pyrethroid insecticides,
Enviromental health perspective, 1980; 34: 189-202.
15.
Burgess IF, Brown CM and Burgess NA; Synergized pyrethrin
mouses a new approach to head lice eradication; Pyrethrum post, Official
publication of Pyrethrum board of keneya 1994; 19; (2): 41-46.
16.
Mbaria JM, Maitho TE, Milema
ES and Muchiri DJ; Comparative efficacy of pyrethrum marc with Albendazole
against sheep gastrointestinal nematodes; Tropical Animal Health and
Production, 1998; 30: 17-22.
17.
Panchal GM, Venkatakrishna Bhat, Devasankaraiah G,
Gopalakrishna G, Patel VK; Anaesthetic activity of Anacyclus Pyrethrum in
laboratory animal; Indian Journal of Pharmacology 2001;33:296.
18.
Bendjeddou D, Lalaoui
K, Satta D, Immunostimulating activity of the hot water-soluble polysaccharide
extracts of Anacyclus pyrethrum, Alpinia galanga and Citrullus colocynthis , J
Ethnopharmacol. 2003; 88 ;(2):155-60.
19.
Sharma V, Thakur M, Singh N, Kumar V ;Evaluation of the Anabolic, Aphrodisiac and
Reproductive Activity of Anacyclus Pyrethrum DC in Male Rats Scientia
Pharmaceutica, 2009; 77; 97–110.
20.
Badhe SR, Badhe RV,
Ghaisas MM, Chopade VV, Deshpande AD.;Evaluations of antidepressant activity of
Anacyclus pyrethrum root
extract; International Journal of Green Pharm 2010;4:79-82.
21.
Gautam OP, Savita Verma, Jain SK, Anticonvulsant and Myorelaxation activity of Anacyclus
Pyrethrum DC. Root extract, Pharmacologyonline 2011; 1: 121-125.
22.
Sujith K, Suba V and
Ronald DC; Neuropharmacological
profile of ethanolic extract of Anacyclus pyrethrum in Albino wistar rats,
IJPSR, 2011; 2; (8): 2109-2114.
23.
Chaouki S, Benali O, Boufeldja T,
Lahcen L, Yahia H, Green corrosion inhibitor: inhibitive action of aqueous extract of Anacyclus pyrethrum L. for the corrosion of mild steel in
0.5 M H2SO4, J. Mater. Environ.
Sci. 2012; 3(1): 206-219.
24.
Sukumaran K, Kuttan R
Inhibition of tobacco-induced
mutagenesis by eugenol and plant extracts available at http://www.ncbi.nlm.nih.gov/pubmed/7753104
accessed on 5 march 2012.
25.
Antnthanarayan and Paniker, Text book of microbiology,
Orient longman, Hyderabad, 2009; 8: 195-199.
26.
Harris L, Foster SJ and Richards RG, An introduction to
staphylococcus aureus and techniques for identifying and quantifying s.aureus
adhesins in relation to adhesion to biomaterial, 2002; 4: 39-60.
27.
Antnthanarayan and Paniker, Text book of microbiology, Orient
longman, Hyderabad, 2009; 8: 270-281.
28.
Antnthanarayan and Paniker, Text book of microbiology, Orient
longman, Hyderabad 2009; 8: 315-317.
29.
David pollack, Student presentation on Salmonella entrica
typhi, University of connecticut, Department of molecular and cell biology; 2003.
30.
Proteus microbilis, Technical information, Manson chemical
Company 2010; 1-9.
31.
Stephen PD, Norman AH, Sean PG, Hugo and Russell’s, Pharmaceutical
Microbiology, Blackwell Science Malden, 2004; 7: 119-131.
32.
Allen DA, Austin B, and Colwell RR;
Antibiotic resistance patterns of metal-tolerant bacteria
isolated from an estuary, Antimicrob Agents Chemother, 1977; 12:545-547.




















No comments:
Post a Comment